CRISPR Therapeutics AG, a leader in gene-based medicines, has recently released its financial results for the second quarter ending June 30, 2024. The company’s financial performance reflects both challenges and significant progress across its therapeutic pipeline. Here, we delve into the financials, highlight key developments in their gene therapy programs, and provide a visual representation of their performance.
Financial Performance
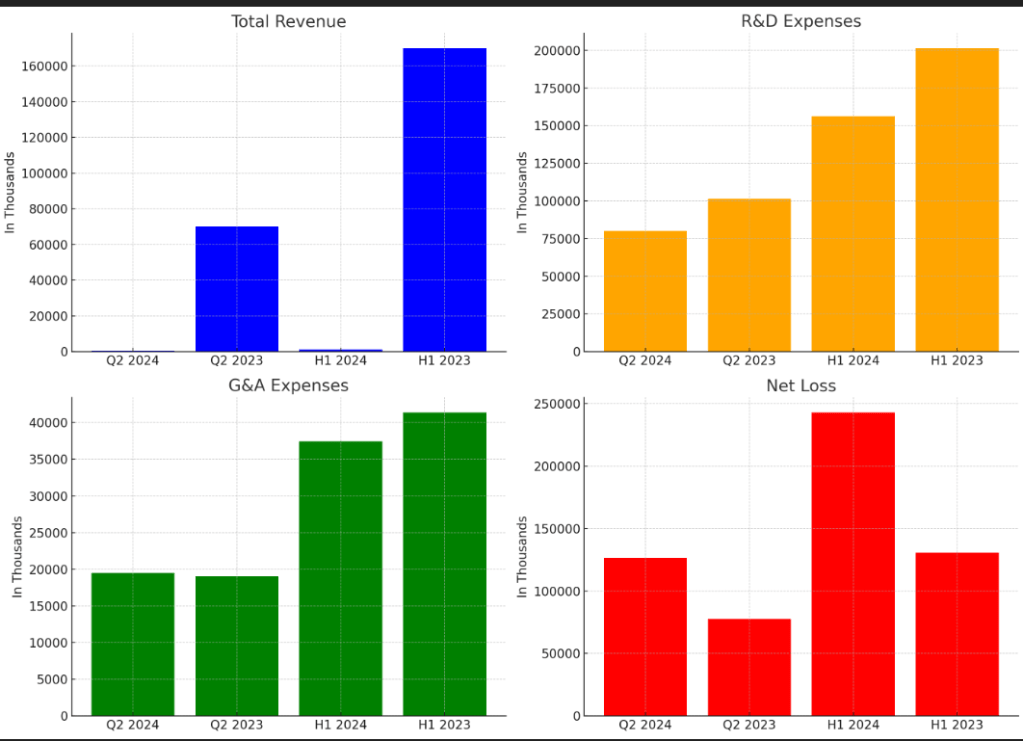
Total Revenue
- Q2 2024: $517,000
- Q2 2023: $70 million
- H1 2024: $1.021 million
- H1 2023: $170 million
The decline in revenue is primarily due to the absence of milestone achievements in the current period.
Research and Development (R&D) Expenses
- Q2 2024: $80.2 million
- Q2 2023: $101.6 million
- H1 2024: $156.3 million
- H1 2023: $201.5 million
R&D expenses have decreased, driven by reduced external research and manufacturing costs.
General and Administrative (G&A) Expenses
- Q2 2024: $19.5 million
- Q2 2023: $19.0 million
- H1 2024: $37.4 million
- H1 2023: $41.4 million
G&A expenses have remained relatively stable over the periods.
Net Loss
- Q2 2024: $126.4 million
- Q2 2023: $77.7 million
- H1 2024: $243.0 million
- H1 2023: $130.8 million
The net loss has increased due to the decline in revenue and the rise in collaboration expenses.
Cash Position As of June 30, 2024, CRISPR Therapeutics held $2.0128 billion in cash, cash equivalents, and marketable securities, compared to $1.6957 billion as of December 31, 2023. This increase is driven by a $280 million direct offering, a $200 million milestone payment from Vertex, proceeds from employee option exercises, and interest income, offset by operating expenses.
Key Pipeline Developments
CASGEVY™ (exagamglogene autotemcel [exa-cel]) CASGEVY is a groundbreaking gene-based therapy targeting sickle cell disease (SCD) and transfusion-dependent beta thalassemia (TDT). Here are the highlights:
- Treatment Centers and Patient Enrollment: Over 35 authorized treatment centers (ATCs) have been activated globally, with approximately 20 patients having cells collected across all regions as of mid-July.
- Clinical Trials: Enrollment is complete in two global Phase 3 studies for patients aged 5 to 11 with SCD or TDT. Positive long-term data from the CLIMB-111, CLIMB-121, and follow-up studies were presented, demonstrating durable clinical benefits.
- Regulatory Approvals: CASGEVY is approved in the U.S., Great Britain, the European Union, Saudi Arabia, and Bahrain for treating SCD and TDT. Regulatory submissions have been completed in Switzerland and Canada, where it received Priority Review.
Oncology and Immunotherapy Candidates
CTX112™
- Target: CD19-positive B-cell malignancies.
- Current Phase: Phase 1/2 trials in oncology settings and clinical trials for systemic lupus erythematosus (SLE).
- Potential: Expansion into autoimmune indications due to effective depletion of B cells.
CTX131™
- Target: CD70 in hematological malignancies, including T-cell lymphomas.
- Current Phase: Ongoing clinical trials in solid tumors and hematologic malignancies.
CTX310™ and CTX320™
- Targets: ANGPTL3 and LPA, respectively.
- Current Phase: Ongoing Phase 1 trials for cardiovascular disease-associated conditions.
CTX211™
- Focus: Allogeneic, hypoimmune, gene-edited stem cell-derived product for Type 1 Diabetes.
- Current Phase: Ongoing Phase 1 clinical trial.
Remaining Therapies
Preclinical Programs
- CTX340™: Targeting angiotensinogen for refractory hypertension.
- CTX450™: Targeting 5’ aminolevulinic acid synthase for acute hepatic porphyrias.
Regenerative Medicine
- CTX211™: Beta-cell replacement product for Type 1 Diabetes, aiming to eliminate the need for chronic immunosuppression.
Conclusion
CRISPR Therapeutics continues to advance its pipeline despite the financial challenges faced in Q2 2024. The company’s strong cash position supports ongoing and future research, reinforcing its commitment to bringing transformative medicines to patients. With numerous clinical trials underway and significant developments in gene-based therapies, CRISPR Therapeutics remains a key player in the biopharmaceutical landscape.
Stay tuned for more updates on their progress and the potential impact of their innovative therapies.




Leave a comment